Further confirmation of the validity of PHMB, currently in development for the treatment of Acanthamoeba keratitis.
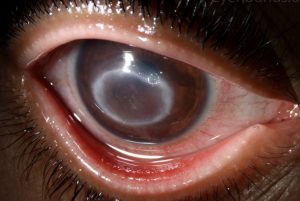
After the European designation (EU/3/07/498), the Food and Drug Administration (FDA) also recognised orphan drug status (orphan drug) with PHMB (Polyhexamethylene Biguanide) for the treatment of Acanthamoeba keratitis, a serious corneal disease caused by the protozoan Acanthamoeba spp.
The designation of PHMB as an orphan drug was granted to SIFI, the Italian ophthalmic society leading the ODAK Project (Orphan Drug for Acanthamoeba Keratitis), a research project funded by the European Commission, which aims to develop a PHMB-based eye drops for the effective and safe treatment of Acanthamoeba Keratitis.
The Acanthamoeba keratitis is a rare disease ('orphan disease'), which affects less than 0.1 in 10,000 EU citizens. Acanthamoeba is a microbial protozoan with a life cycle consisting of two main phases: trophozoite and cyst. While the former is sensitive to the most common chemotherapeutic agents, the latter is an inactive form that can survive in extreme environmental conditions.
Patients with Acanthamoeba keratitis generally experience severe eye pain, photophobia, redness and irritation of the eye, blurred vision and excessive tearing.
Clinical signs include annular stromal infiltrates, epithelial defects and eyelid oedema. Contact lens wearers are particularly at risk and account for up to 85 % of CA cases. Patients often present with very severe forms of Acanthamoeba infection and about 251 % of them have to resort to corneal transplantation.
A multicentre clinical trial is being conducted in Europe. In the clinical study, a solution of PHMB 0.08% is compared with the most widely used off-label treatment today, consisting of PHMB 0.02% in combination with Propamidine 0.01%. The study has already recruited 18 patients out of a total of 130 and is scheduled to conclude at the end of 2018.
For more information visit http://www.odak-project.eu/
Dr. Carmelo Chines
Direttore responsabile
