A small chemical modification extends the duration of effectiveness of drugs
A small chemical modification extends the duration of effectiveness of drugs
One, two or three drops of an eye drop?
How many doses per day are needed for each drug?
The answer lies, among other things, in the processes of metabolic clearance, and it can also happen that some of the active substances in a drug are eliminated before they have had a chance to produce their full effect.
Remember that eye drops are a specific pharmaceutical formulation, specifically a liquid solution, usually based on a solvent, which can be water.
Eye drops are administered into the eye to treat or relieve eye disorders, such as conjunctivitis, or to soothe symptoms such as red eyes or dryness.
The 'stratagems'
 Is there any trick to prolong the effectiveness of eye drops?
Is there any trick to prolong the effectiveness of eye drops?
The answer is yes and consists of a 'trick' that intervenes in the atomic structure, without changing the properties of the product.
But let us look in more detail. As is well known, the chemical properties of an atom are determined by its electrons, which interact with those of other atoms.
The electrons are equal in number to the protons that make up the nucleus, which as a whole has an electrical charge of 0 because the protons have a positive charge, while the electrons have a negative charge.
The number of electrons uniquely identifies a chemical 'element'. In the nucleus, however, in addition to electrons there are neutrons, which do not possess any electrical charge.
Atoms of the same element may have different numbers of neutrons, which results in a slight variation in their mass, but generally does not alter their chemical properties.
The only exception is thehydrogenIf a neutron is added to the normal hydrogen atom, we obtain the deuterium, which has important differences in terms of chemical bonds.
Deuterium
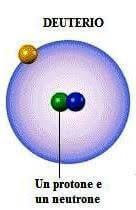
The deuterium (from ancient greek: δεύτερος?, déuteros, 'second') is a isotope stable of thehydrogen whose core (called deuteron or deuteron) is composed of a proton and a neutron. Although it is not really a chemical element in its own right, the symbol 2H o D to indicate this.
It was discovered in 1931 by Harold Ureywho won the Nobel Prize in Chemistry in 1934.
His isotopic abundance is 0.015% (0.030% in terms of mass). As the most common hydrogen isotope, the great uncleAt room temperature and pressure, deuterium forms a gas of molecules diatomic with the formula 2H2 or D2. On earth it occurs naturally in minute percentages in the water molecule, from which deuterium-enriched water can be produced artificially, theheavy water.
Properties
Deuterium forms stronger bonds with carbon atoms than normal hydrogen. In many cases, metabolic clearence depends precisely on the breaking of particular carbon-hydrogen bonds.
Biochemical aspects
Replacing hydrogen atoms with deuterium in certain drugs can therefore slow down metabolic clearence and thus ensure that they remain active for longer.
How it affects durability
Deuterium can affect the efficacy of drugs, particularly their duration of action.
Replacing hydrogen with deuterium in some pharmaceutical molecules can slow down metabolism, prolonging the drug's activity in the body.
This occurs because deuterium has a lower tendency to be exchanged or eliminated than hydrogen, thus affecting the rate at which the drug is metabolised and eliminated.
A bit of history
In fact, this possibility had been known about since the 1970s, but it was not until the mid-1990s that 'deuterised' versions of some already registered drugs came onto the market. Initially, deuterised versions succeeded in obtaining a new patent, but in the case of more recently registered drugs, 'deuterisation' is generally already included in the first patent, and the US patent office began to reject patent applications for deuterised versions of existing drugs.
Perspectives
Clearly, it is still to be clearly defined what will be considered 'innovative' and what will be considered 'obvious' for existing drugs.
Benefits for patients
What is beyond doubt is that patients will benefit greatly from having fewer pills to swallow or eye drops to instil!
On the same subject read the article published in "The Economist' of 5 September.
On the subject of eye drops see also:
