Angioid striae represent continuous solutions or dehiscences of Bruch's membrane, which is thickened, calcified and abnormally fragile.
The first description of striae dates back to 1889 when Doine described this aspect of the fundus oculi in a patient who presented with a post-traumatic retinal haemorrhage. The term 'angioids' was instead coined by Knapp who hypothesised a vascular origin of the pathology. Later Kofler in 1917 guessed that the angioid striae had little to do with the vessels, but were also changes in Bruch's membrane. Further clinical observations, corroborated by histopathological data published in the late 1930s, confirmed the substantial correctness of this hypothesis.
Epidemiology and pathogenesis
Although angioid striae (AS) have never been observed at birth, they have been documented in childhood. They represent continuous solutions or dehiscences of Bruch's membrane, which is thickened, calcified and abnormally fragile.
It has been hypothesised that the striae may be the consequence of even minor trauma or that they are related to the lines of force transmitted by the eye muscles at the level of the NO. It seems more likely that they occur spontaneously due to a primitive metabolic alteration involving the elastic fibres of Bruch's membrane. However, the pathogenetic mechanisms causing these alterations are unclear. The prevalence of AS is unknown; there are no differences in incidence in relation to race or gender.
Ophthalmoscopic appearance
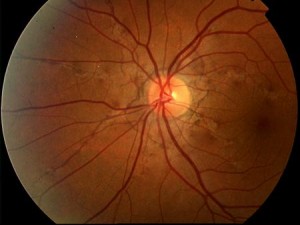
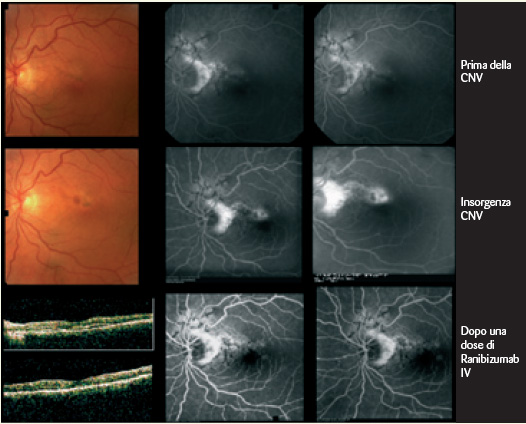
AS manifest themselves as thin, jagged reddish-brown lines radiating from the optic nerve and mimicking the course of subretinal vessels (Figs. 1-2).
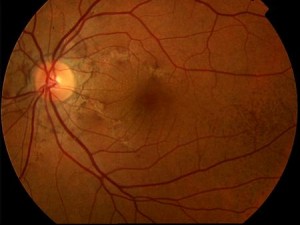
The striae are normally bilateral although asymmetry between the two eyes is the rule. In addition to the more frequent radial pattern, AS sometimes take on the appearance of incomplete, irregular peripapillary circular rings. More rarely they may assume a random arrangement at the posterior pole. They are always separated from the optic disc and may extend far anteriorly. Serious observations have shown that the AS extend with time. The colour of the AS is determined by both the pigmentation of the fundus and the degree of atrophy of the EPR overlying the lesions. Pigmentary changes of the EPR, characterised by focal accumulations, are often evident at the margins of the striae. The fundus may be characterised by a diffuse pigmentary alteration, often localised temporally to the macula, termed 'orange peel'. This alteration would be more frequent, but not exclusive, in patients with Pseudoxanthoma Elastica (PXE).
In approximately 25% of patients with AS, there are associated drusen of the papilla that can be detected ophthalmoscopically or ultrasound.
Diagnosis
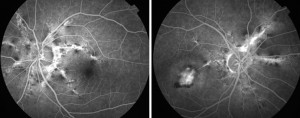
The diagnosis of AS is often obvious and only rarely is the comfort of a fluorangiographic (FA) examination required, as the lesions may be barely perceptible ophthalmoscopically. On FA the striae are variously hyperfluorescent (by transmission) depending on the state of the EPR above the lesions. At the margins of the striae pigment accumulations may be evident resulting in mask effect hypofluorescence. If a CNV is associated, the angiographic appearance is generally that of a classic membrane although occult membranes have been described in AF (Figs. 3-4).
Differential diagnosis
The differential diagnosis must be made with the pathologies listed in the table and does not normally pose particular difficulties.
TABLE
- Choroid ruptures
- Lacquer cracks
- Myopic degeneration
- CNV in age-related macular degeneration
Systemic associations
The systemic pathologies associated with AS are listed in the table; for the sake of brevity, we will deal only with the most frequent association (Pseudoxanthoma Elastica), referring to the literature for a more in-depth discussion of the other systemic pathologies.
- Pseudoxanthoma Elastica (PXE)
- Paget's disease
- Ehlers- Danlos Syndrom (Type 6)
- Haemoglobinopathies
The most common systemic disease associated with AS is PXE . In one of the largest case series, approximately 50% of patients with AS were carriers of PXE. PXE is a genetic disorder characterised by the progressive calcification and fragmentation of elastic fibres of connective tissue that affects the cardiovascular, digestive and uro-genital systems in addition to the skin and choriocapillary. The disease is transmitted in both an autosomal dominant and recessive manner; there is no gender or racial predilection. Characteristic changes in the skin and subcutaneous tissue are most often evident in the neck, armpits, groin and periumbilical area (orange peel skin). Typically the skin lesions begin in childhood, but being asymptomatic they are only recognised in adolescence. The main systemic manifestations of PXE occur in the chorioretina (striae + CNV), the digestive (haemorrhages) and cardiovascular system (valvulopathies) and the genito-urinary tract (haematuria - haematospermia).
The pathophysiology of the disease is characterised by a genetically determined primitive disorder of the elastic tissue, evidenced at skin level in histological section. Haematoxylin-eosin staining shows the characteristic 'basophilia' of the elastic fibres caused by calcium deposition. In the intermediate and deep dermis the elastic fibres appear fragmented, swollen and clustered. Similar changes can be seen in the tunica media and in the intima of the blood vessels and at the level of Bruch's membrane.
Indocyanine green angiography (ICGA), as it is able to pass the EPR filter, shows that hyperfluorescent striae are wider and more numerous than those seen with AF. ICGA better defines the presence of CNV in the case of AF-blocked fluorescence.
Therapy
The prognosis of AS is always reserved and central visual function is severely impaired in at least 70% of patients. This occurs not only due to the onset of CNV, but also due to choroid rupture caused by even mild trauma or the extension of striae to directly involve the fovea.
Patients with AS must therefore be instructed to avoid potentially traumatic activities due to the danger of the onset of retinal haemorrhage or choroid rupture.
The treatment of CNV associated with SA has always represented a particular challenge for ophthalmologists, given the poor prognosis of the condition.
The treatment options, which have changed in parallel with those of CNV associated with AMD, are:
- Direct laser treatment of extrafoveal shapes
- Photodynamic therapy with verteporfin
- Photodynamic therapy combined with triamcinolone IV
- IV injection of VEGF inhibitors
The efficacy of laser photocoagulation has never been verified in controlled clinical studies. There is sporadic evidence that the treatment of extra or juxta foveal forms may be of some efficacy. Singerman and Hatem reported good long-term results in seven out of eight patients. The most extensive experience with photocoagulative treatment is reported by Pece et al. who stabilised visual acuity in a series of 66 eyes of 52 patients with classic extrafoveal CNV. The limitation of this therapeutic procedure is obviously the impossibility of treating subfoveal forms and the very high recurrence rate estimated at around 77%. Moreover, the natural evolution of CNV in patients with AS would appear to be particularly severe, as reported in a brief case history by Lim et al. who in a group of 11 untreated patients found final visus in all of them to be counting fingers.
In addition, unsuccessful attempts have been made to slow down the progression of striae towards the fovea by means of prophylactic laser photocoagulation, which, in addition to being useless, can in itself stimulate the formation of a CNV.
Photodynamic therapy with Verteporfin (PDT-V)
As with direct laser treatment, there are no controlled clinical studies that have verified the safety and efficacy of PDT-V in patients with CNV secondary to SA. Photodynamic treatment therefore represents a rational but arbitrary extension made by analogy to the treatment of the senile and myopic form of CNV.
The information available in the literature is scant and contradictory. It consists of small series of cases in which encouraging results alternate, consisting of stabilisation of the pathology, and less good results, characterised by progression of the lesion and progressive reduction of visual acuity. The largest case series produced in the literature is that of Menchini, who cumulated the results obtained in 6 Italian centres out of 48 eyes, highlighting a progressive extension of CNV in over 60% of patients treated and a reduction in visual acuity in approximately 50% of patients.
Other limited series have reported substantially similar results, and an overall review of these case reports suggests that PDT does not appear to be a good treatment for striae-associated CNVs because, the benefits are transient, relapses are frequent and the long-term prognosis does not appear to be affected by the therapy.
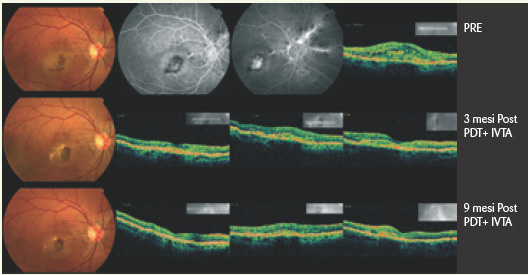
The combination of PDT-V with intravitreal injection of triamcinolone also yielded controversial effects, with alternating surprising results (Fig. 5) and less encouraging ones.
VEGF inhibitors
The recent introduction of VEGF inhibitors in the treatment of CNV opens up new horizons in the treatment of the SA-associated form. A few papers on small case series have recently appeared in the literature suggesting the efficacy of VEGF inhibitors in this disease. These initial encouraging results will have to be verified over time, given the particular tendency of CNV associated with SA towards progression and relapse. Episodic personal observations seem however to confirm the possibility of good results, at least in the short term (6).
Federico Ricci
UOSD Retinal Pathologies
Polyclinic Foundation
Tor Vergata, Rome
Bibliography
1. Mansour AM, Shields JA, Annesley WH Jr, et al. Macular degeneration in angioid streaks. Ophthalmological 1988;197:36-41.
2. Clarkson JG, Altman RD. Angioid streaks. Surv Ophthalmol 1982;26:235-46.
3. Singerman LJ, Hatem G. Laser treatment of choroidal neovascular membranes in angioid streaks. Retina 1981;1:75-83.
4. Pece A, Avanza P, Galli L, Brancato R. Laser photocoagulation of choroidal neovascularization in angioid streaks. Retina 1997;17:12-16.
5. Menchini U, Virgili G, Introini U, et al. Outcome of choroidal neovascularization in angioid streaks after photodynamic therapy. Retina 2004;24(5):763-771.
6. Marco Zarbin. Should corticosteroids be considered as part of the standard care with photodynamic therapy? Am J Ophthalmol 2006;124:563-571.
7. Spaide R, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology 2003;110:1517-1525.
8. Ciulla TA, Criswell MH, Danis RP, Hill TE. Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch Ophthalmol 2001;119:399-404.
9. Gillies MC, Simpson JM, Luo W, et al. A randomised clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: one-year results. Arch Ophthalmol 2003;121:667-673.
10. Spaide RF, Sorenson J, Maranan L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology 2005;112:301-304.
11. Arias L,Garcia-Arumi J, Ramon J.M., et al. Photodynamic Therapy with Intravitreal Triamcinolone in Predominantly Classic Choroidal Neovascularization. One-Year Results of a Randomized Study Ophthalmology 2005;112(7):1227-1231.
12. Kaiser P.K. Verteporfin therapy in combination with triamcinolone: published studies investigating a potential synergistic effect. Curr Med Res Opin 2005;21(5):705-713.
13. Sandowski T, Steinmeyer J. Effects of polysulfated glycosaminoglycan and triamcinolone acetonid on the production of proteinases and their inhibitors by IL-1alpha treated articular chondrocytes. Biochem Pharmacol 2002;15;64(2):217-227.
14. Wang YS, Friedrichs U, Eicler W, et al. Inhibitory effects of triamcinolone acetonide on bFGF- induced migration and tube formation in choroidal microvascular endothelial cells. Graefes Arch Clin Exp Ophthalmol 2002;240:42-48.
15. Gordon SG, Overland JF, Foley J. Evidence for increased protease activity secreted from cultured fibroblasts from patients with pseudoxanthoma elasticum. Conn Tissue Res 1978;6:61-68.
16. Schwartz, F.A. Cruickshank, M.G. Lebwohl, Elastase-like protease and elastolytic activities expressed in cultured dermal fibroblasts derived from lesional skin of patients with pseudoxanthoma elasticum, actinic elastosis, and cutis laxa. Clin Chim Acta 1988;176:219-224.
17. Quaglino D, Sartor L, Garbisa S, et al. Dermal fibroblasts from pseudoxanthoma elasticum patients have raised MMP-2 degradative potential. Biochim Biophys Acta 2005 Jun 30;1741(1-2):42-47.
18. Teixeira A, Moraes N, Farah ME, Bonomo PP. Choroidal neovascularization treated with intravitreal injection of bevacizumab (Avastin) in angioid streaks. Acta Ophthalmol Scand 2006;84(6):835-836.
Dr. Carmelo Chines
Direttore responsabile