Glaucoma continues to be one of the leading causes of irreversible blindness worldwide, often because it is diagnosed when optic nerve damage is already advanced. Despite therapeutic advances, a complete understanding of the molecular mechanisms underlying the disease is still lacking.
In recent years, knowledge and identification of the micro-RNA (miRNA) - small regulatory molecules that finely modulate gene expression - are proving to be powerful tools for understanding how glaucoma develops, progresses and responds to treatment.
The idea that tiny RNA fragments can revolutionise diagnosis and therapy represents a promising new vision in modern ophthalmological medicine.
Glaucomatous optic neuropathy
 Glaucoma is a chronic degenerative disease affecting the optic nerve, characterised by damage to its nerve fibres and consequent visual field damage. If left untreated, the progressive reduction of the visual field can lead to blindness.
Glaucoma is a chronic degenerative disease affecting the optic nerve, characterised by damage to its nerve fibres and consequent visual field damage. If left untreated, the progressive reduction of the visual field can lead to blindness.
Glaucoma is a major social problem: it is the second leading cause of blindness in the world and affects about 60 million people, rendering more than 8 million of them blind. Moreover, about 50% of glaucoma sufferers are unaware of it.
It is a sneaky disease as one often notices the disease when visual changes are already very advanced, whereas previously one did not experience any symptoms. However, if diagnosed early and properly treated, it can be effectively kept under control, allowing good vision throughout life.
Micro-RNA and Glaucoma
Glaucoma is actually not a single disease, but a group of complex conditions involving numerous biological, genetic and environmental factors. For this reason, the possibility of having precise molecular markers and key regulatory mechanisms is a huge step forward.
Micro-RNAs could become those invisible 'interpreters' that tell what is really going on inside the cells of the optic nerve, retina and trabeculae, providing valuable clues about the health of the eye well before symptoms appear.
The role of Micro-RNA
Micro-RNAs act as precision regulators within the cell: they modulate the expression of hundreds of genes, control protein production and maintain the balance of vital processes such as inflammation, oxidative stress, apoptosis and metabolism.
They bind to messenger RNA, acting as a 'filter' that decides which messages should be translated into protein and which should be silenced.
A number of micro-RNA tracts that perform specific functions that may contribute to the onset of glaucoma have been identified, in detail:
- increase apoptosis of retinal ganglion cells
- affect the production or outflow of aqueous humour
- modulate the inflammatory response of the optic nerve
- play a role in changes in intraocular pressure (IOP)
Identifying which micro-RNAs are altered can, therefore, provide a detailed reading of ongoing disease processes.
Precisely because glaucoma is insidious and many patients do not perceive symptoms until the late stages, there is great interest in finding reliable biomarkers that can reveal the disease at the stages when it is still silent.
Micro-RNA detection can be performed using a variety of body fluids:
- blood
- tears
- aqueous humour
- retinal tissues
There is, therefore, the possibility of finding micro-RNAs through a non-invasive and early approach, based on the detection of molecular alterations, which precede structural damage observable by OCT or visual field.
Micro-RNAs could, therefore, function as 'early warnings', capable of identifying at-risk patients long before vision is impaired.
Biogenesis and Mechanisms of Micro-RNA
To use micro-RNAs as diagnostic or therapeutic tools, it is essential to know their biology, from their origin to their action on ocular tissues.
The micro-RNA pathway starts in the cell nucleus, where DNA is transcribed into a long precursor called pri-miRNA.
Subsequently:
- Droshaa key enzyme, cuts pri-miRNA into pre-miRNA.
- The pre-miRNA is transported into the cytoplasm via the protein Exportin-5.
- Diceranother essential enzyme, further refines the molecule.
- The mature micro-RNA is loaded into the complex RISCready to perform its regulatory function.
Drosha is part of a protein complex called Microprocessor complex (microprocessing complex), which contains double-stranded RNA attachment proteins that are essential for Drosha to be able to attach to the double-stranded RNA fragments of the pri-miRNA that are required for proper processing, as these are sensors of the RNA's flexibility and can recognise the right attachment point of the complex.
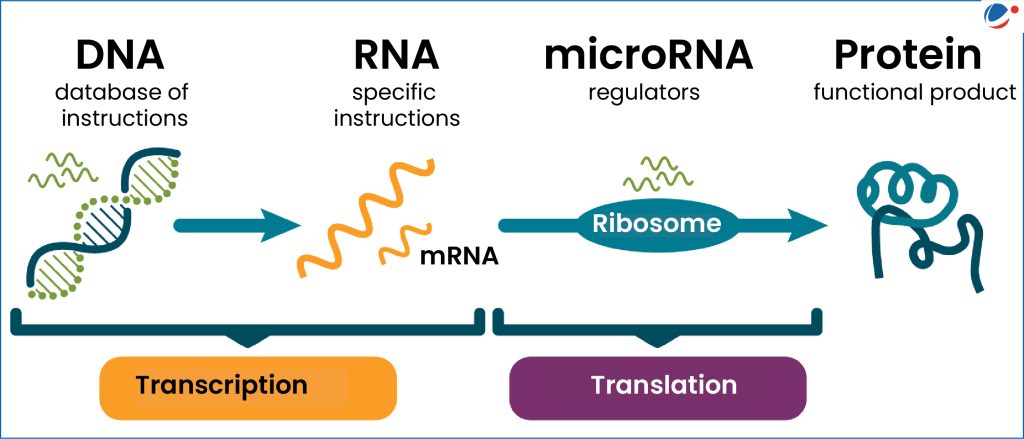
Each step can be influenced by environmental stimuli and pathological conditions - including ocular hypertension - altering the availability of active micro-RNAs in tissues.
Gene Silencing Mechanisms
Once activated, micro-RNA recognises specific sequences on messenger RNA (mRNA). This interaction can:
- block translation into protein
- promote mRNA degradation
- reduce the efficiency of cellular processes that depend on that protein
In glaucoma, this process is closely linked to mechanisms such as:
- weakening of ganglion cells
- alterations of the trabecularis
- imbalances in aqueous humour production
- oxidative damage to the optic nerve
Micro-RNA profiling in Glaucoma
To study how micro-RNAs change in glaucoma, advanced technologies are used that allow great sensitivity and precision.
Real-Time PCR and ddPCR
The Real-time PCR (qPCR) allows the quantity of a micro-RNA to be measured with great accuracy by amplifying very weak signals.
The droplet digital PCR (ddPCR) represents a further development, splitting the sample into thousands of micro-droplets and offering:
- absolute quantification
- higher sensitivity
- less variability between tests
These methods make it possible to identify even minute changes in micro-RNA associated with changes in ocular pressure or optic nerve damage.
Small RNA Sequencing and Reporter Assay
Small RNA sequencing makes it possible to discover the entire 'landscape' of micro-RNAs in a sample, including rare or hitherto unknown ones.
The assay with reporterInstead, they directly measure the micro-RNA → mRNA interaction, visualising the specific targets involved in glaucoma.
These combined tools make precise mapping of the molecular circuits of the disease possible.
Advantages of Micro-RNA in the Treatment of Glaucoma
Once the micro-RNAs involved are known, it becomes possible to imagine new therapeutic strategies based on the modulation of their activity.
Biomarkers for Early Diagnosis
Alterations in micro-RNA levels could allow diagnosis:
- open-angle glaucoma
- congenital glaucoma
- early progression of the damage before the retinal ganglion cells begin to die.
A simple tear sample, for example, could one day become part of routine screening.
Personalised Medicine and Therapeutic Potential
The micro-RNA profile of each patient could reveal:
- individual risk of progression
- response to treatments such as prostaglandins or beta-blockers
- susceptibility to side effects
- biological pathways most active in the individual eye
This will enable truly tailor-made therapies.
In the future, we may even have:
- micro-RNA mimics to restore protective functions
- micro-RNA inhibitors to block harmful pathways
This type of development could mark a new era in optic nerve neuroprotection.
Challenges and Limits of Micro-RNA Research
Despite its great potential, the clinical integration of micro-RNAs still presents major obstacles.
Variability and Lack of Standardisation
A micro-RNA approach, unfortunately, still suffers from the lack of uniform protocols for:
- sample collection
- storage of ocular fluids
- methods of extraction
- analysis platforms
The absence of uniform protocols makes it difficult to compare results obtained in different laboratories. Standardisation at international level will be essential to make micro-RNAs reliable clinical tools.
Limited Understanding of Functional Meaning
Many micro-RNAs have been identified as altered in glaucoma, but their exact role in the onset of the disease remains unclear.
The complexity of the gene networks involved makes it difficult to attribute a unique effect to each micro-RNA.
Large-scale longitudinal studies are needed to distinguish:
- disease-causing micro-RNA
- micro-RNA consequence of the disease
- micro-RNAs only marginally involved
A Promising Future
In summary, micro-RNAs represent a new and promising frontier for the diagnosis, understanding and treatment of glaucoma and ocular hypertension.
With the evolution of profiling techniques and more numerous and structured clinical studies, we may soon witness a radical transformation of the diagnostic and therapeutic approach.
The future of glaucoma treatment could be written in sequences as short as 22 nucleotides!
- Greene KM, Stamer WD, Liu Y. The role of microRNAs in glaucoma. Exp Eye Res. 2022 Feb;215:108909. doi: 10.1016/j.exer.2021.108909. Epub 2021 Dec 27. PMID: 34968473; PMCID: PMC8923961
- Dobrzycka M, Sulewska A, Biecek P, Charkiewicz R, Karabowicz P, Charkiewicz A, Golaszewska K, Milewska P, Michalska-Falkowska A, Nowak K, Niklinski J, Konopińska J. miRNA Studies in Glaucoma: A Comprehensive Review of Current Knowledge and Future Perspectives. Int J Mol Sci. 2023 Sep 28;24(19):14699. doi: 10.3390/ijms241914699. PMID: 37834147; PMCID: PMC10572595.
